Remote Monitoring of Ovulation and Pregnancy of Yellowstone Bison
J. F. Kirkpatrick, Deaconess Research Institute, 1500 Poly, Billings, Mt 59102
D. F. Gudermuth, Department Of Psychology, Cornell University, Ithaca, NY 14850
R. L. Flagan, Department Of Biological Sciences, Eastern Montana College, Billings, Mt 59101
J. C. Mccarthy, Department Of Biological Sciences, Eastern Montana College, Billings, Mt 59101
B. L. Lasley, Institute for Toxicology and Environmental Health, University of California, Davis, CA 95616
Reprinted from the Journal of Wildlife Management, 57(2):1993
Abstract:
The physiological mechanisms that control reproductive success of wild bison (Bison bison) are not known, and the relatively small scattered herds prevent intensive study. Environmental, demographic, and physiological factors all play major interrelated roles in reproductive self-regulation. Thus, we validated the use of urinary and fecal steroid analysis as a means of detecting ovulation and pregnancy in uncaptured free-roaming ungulates, and identified the physiologic mechanisms that govern reproductive success in wild bison. Free-roaming bison of 2 subpopulations of the Yellowstone National Park herd were observed during 1989-91. Ovulation was detected by the measurement of urinary pregnanediol-3-glucuronide and fecal progesterone during the rutting season; pregnancy was detected by increased urinary estrone conjugates and fecal total estrogens during the third month of gestation. Among 54 sexually mature cows observed being tended (showed clinical signs of estrus and constantly attended by a bull) during the rutting season, 18.5% were lactating, and 81.4% were not lactating. The documented ovulation rate for 121 mature cows during the same period was only 14.5% among lactating cows. The estimated pregnancy rate over 2 years for 255 mature cows was 48.2%; 15.4% of the pregnancies were among lactating cows. Our data suggest that approximately 85% of all mature cows are pregnant on alternate years, approximately 15% of lactating cows are fertile, the cause of lowered fertility in lactating cows is lactational anovulation, and endocrine evidence of ovulation and pregnancy, based on urinary and fecal steroids is consistent with all other observed reproductive behaviors.
The North American bison has been studied extensively with regard to social organization (McHugh 1958), behavior (Rutberg 1984a, Green 1986, Lott and Galland 1987), and habitat use (Norland 1984). Reproductive studies largely have been confined to descriptions of behavior (Lott 1981; Rutberg 1984b, 1986; Maher and Byers 1987) or population patterns (Meagher 1989). The rutting season in Yellowstone National Park (YNP) occurs between mid-July and early September. Cows do not normally reproduce until 2 years of age (McHugh 1958) and currently in YNP many do not breed until 3 years (M. M. Meagher, Natl. Park Servo, pers. commun.). The estrous cycle is approximately 3 weeks in duration (Asdell 1964, Kirkpatrick et al. 1991b), with sexual receptivity lasting 1-2 days. Wild free-roaming bison presumably have only a single ovulation per year (Haugen 1974), but Kirkpatrick et al. (1991b) reported a second ovulation among bison in a commercial herd, suggesting that under some conditions bison are seasonally polyestrus.
McHugh (1958) reported that pregnancy rates for sexually mature cows, ages 2-12, varied from 78% to 100% on the National Bison Range (NBR) in Montana, with a significant decrease occurring after age 12. The high overall pregnancy and calving rates for the NBR animals (approx 90% ) are in contrast to the 35% rate reported by Lott and Galland (1987) for an undernourished population on Santa Catalina Island (SCI), California. Meagher (1973) observed that approximately 50% of sexually mature cows produced calves in YNP, and suggested that the majority of cows produced calves every other year. Despite the large and significant differences in range size, population density, nutrition, and weather, little is actually known regarding the precise nature of the physiological mechanisms controlling reproductive success of bison under different environmental conditions. For example, pregnancy rates have been calculated from observed calving rates, and the length of the estrous cycle has been based on behavioral estrus, without any physiological evidence for ovulation.
The current information provides strong evidence that environmental factors have direct effects on the reproductive success of large ungulates such as bison. These data do not, however, provide specific information regarding which physiologic processes of the reproductive system are affected. For example, reduced fertility could be the result of either reduced fecundity of the adults or failure of the embryo or fetus to survive. Thus, we wanted to validate the use of urinary and fecal steroid analysis to remotely monitor ovarian function in free roaming bison, and identify the physiologic mechanisms that govern their reproductive success. Specifically, we examined ovulation rates and pregnancy rates among lactating and nonlactating cows and compared these values with the percent of lactating and non-lactating cows demonstrating estrous behavior and being tended by bulls. Additionally, a sample of tended cows was identified and urine or fecal samples were collected 3-5 days after tending ceased to confirm that cows had actually ovulated.
We thank J. D. Varley and S. E. Broadbent, and M. Meagher of the National Park Service for logistical support in Yellowstone National Park, and helpful advice, C. C. Campbell for help with specimen collections, and D. E. Dilley for technical assistance in analyzing samples. This study was supported by Sigma Xi and the National Science Foundation Conservation and Restoration Biology award DCB-8922800.
Methods and Materials
All data were collected from 2 subpopulations of bison at YNP between November 1989 and September 1991. The 2 subpopulations included the Mary Mountain herd (MM) and the Northern Range herd (NR). The MM herd (about 1,800 animals) is a stable population that inhabits the central regions of the park. The NR herd is distributed throughout the Lamar River Valley and the upper Yellowstone River Valley from Tower Junction to Gardner, Montana, and increased from an estimated 250 in 1980 to about 900 by December 1988. This herd was reduced by a hunt outside the park during the winter of 1988-89, to approximately 300 by May 1989 (Meagher 1989), and subsequently had increased to 520 by September 1991.
Behavioral Observations
Between 1 July and 1 September 1990, we observed and counted bison from that portion of the MM herd residing in the Hayden Valley. Counts included: total bison, sexually mature cows (>2 years), calves, and yearlings. Two-year-old cows were distinguished from yearlings by horn size and shape as described by Fuller (1959). Because cows may group differentially, according to whether they have calves or not, care was taken to include several groups within each subpopulation. We observed cows that were sexually mature and lactating, or mature and non-lactating, throughout the rutting season, and the percentage of each class that displayed estrous behavior (i.e., was being tended by mature bulls) was recorded. Tended cows seldom were observed actually being mounted by a bull, and the few incidences of mounting observed lasted only a few seconds.
Collection of Urine/Fecal Samples
We collected urine or fecal samples from 144 cows inhabiting the Hayden Valley, between 1 July and 1 September 1990. Samples also were collected from 87 and 83 cows of the NR herd in November 1989 and 1990, respectively, and from 85 cows of the MM herd in November 1990. We observed cows at ranges of 50-500 m by means of a spotting scope or binoculars until they voided and the site of a urination or defecation was noted. After the bison had moved a safe distance away, personnel were directed to the site by means of hand-held radios. Urine-soaked soil was collected, packed into the barrel of a 5-cc syringe, stored on ice during the day of collection, and extracted by centrifugation at the end of each day. The 5-cc syringe was placed inside a 15- x 100-mm plastic test tube and cen-trifuged for approximately 15 minutes in a clinical centrifuge (900 x g). The urine was decanted and stored frozen until assayed. Fecal samples were collected and stored as described by Kirkpatrick et al. (1991a ). During November collection periods, urine also was collected by recovering urine-soaked snow as described by Kirkpatrick et al. (1988, 1990b).
Ovulation Detection
The occurrence of ovulation and subsequent luteal function is traditionally based upon a rise in progesterone (P4) in blood as described by Plotka et al. (1967) for cattle. In our study, we assessed changes in P4 concentrations secreted by the ovary via a rise in either urinary pregnanediol-3-glucuronide (PdG) as described in bison (Kirkpatrick et al. 1991a), or fecal P4 concentrations as described in domestic cows (Desaulniers et al. 1989). Among the 144 sexually mature cows in the MM subpopulation from which urine or fecal samples were collected during the rutting season in 1990, 121 were not being tended at the time of collection. Urine samples from these were also assayed for PdG as described by Kirkpatrick et al. (1991a).
We also conducted an experiment to determine if witnessed tending and estrous behavior were reliable indicators of ovulation. We identified 12 tended cows of the NR herd by individual characteristics (scars, horn abnormalities, winter fur patterns, etc.). Fecal samples were collected from each cow 3-5 days following observed tending and analyzed for P4. Ovulation was considered to have occurred if P4 concentrations were >3,000 ng/g dry feces (Kirkpatrick et al. 1992). The intra- and interassay coefficients of variation for the assay were 8.9% (n = 7) and 12.4% (n = 7), respectively. Urinary creatinine (Cr) concentrations were measured by the microcolorimetric method of Taussky (1954) and PdG concentrations were indexed to creatinine and reported in ng/mg Cr to account for differences in urine concentrations. We considered ovulation to have occurred if PdG concentrations were >100 ng/mg Cr (Kirkpatrick et al. 1991a).
After P4 was extracted from fecal samples (after Desaulniers et al. 1989), the extracts were analyzed by enzyme immunoassay (Munro and Stabenfeldt 1984) and validated for bison (Kirkpatrick et al. 1992). The intra- and interassay coefficients of variation were 9.3% (n = 7) and 16.4% (n = 7), respectively.
Pregnancy Detection
We based the occurrence of pregnancy upon the elevation of estrogens during the second month of pregnancy, as originally described in domestic cows (Mostl et al. 1984) and more recently in bison (Kirkpatrick et al. 1992). During November 1989, we collected urine and fecal samples randomly from 87 cows in the NR herd. In November 1990, we collected 83 urine and fecal samples from animals in the same herd, and 85 urine or fecal samples randomly from cows in the MM herd. Urinary estrone conjugates (E1C) were measured by enzyme immunoassay (Shideler et al. 1990) and fecal total estrogens were measured by radioimmunoassay (Kirkpatrick et al. 1990b, 1992). Pregnancy was detected on the basis of E1C concentrations of >10 ng/mg Cr or total estrogen concentrations of >1.0 ng/g dry feces (Kirkpatrick et al. 1992). We compared observed tending rates and estimated ovulation and pregnancy rates, based on urinary and fecal hormone concentrations among lactating and non-lactating cows. No statistical comparisons were made because we did not expect the 2 groups to be the same.
Results
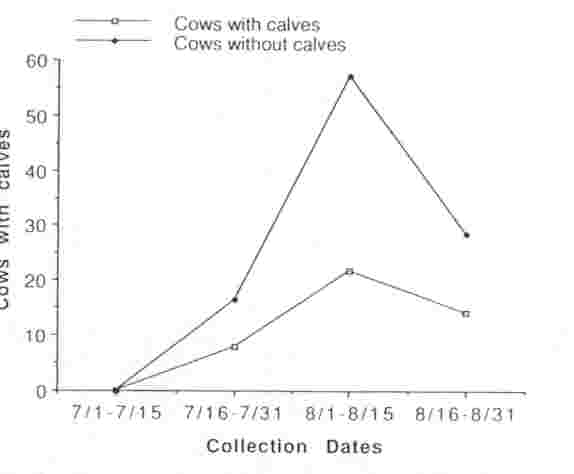
We observed a total of 524 sexually mature cows in 10 different groups within the MM herd between 15 July and 31 August 1990; 45.9% (range = 13.7-58.8%) had calves at their sides. During the peak period of breeding activity (15 Jul and 31 Aug), 54 sexually mature cows were observed being tended by a bull. Of those, 10 (18.5%) had calves at their sides and 44 (81.4%) did not, indicating that estrus, and possibly ovulation, was occurring predominantly among non- lactating cows (Fig. 1). Urine or fecal samples collected from 121 cows that were not being tended at the time of collection revealed 62 (51%) were lactating, and 9 of these (14.5%) had elevated PdG or P4 concentrations indicating that they had ovulated; 59 (49%) were without calves, and 25 (42.3%) of these had elevated PdG or P4 concentrations indicating that they had ovulated. By 1 September, ovulation occurred in non-lactating cows at greater than twice the rate than in lactating cows (Fig. 2).
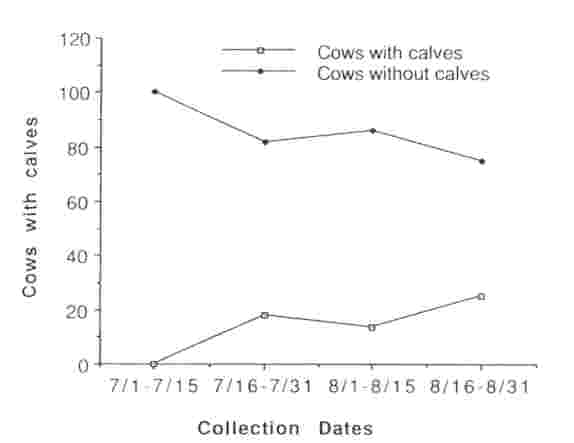
Fig. 1 Proportion of lactating (with calves) and non-lactating (no calves) bison cows that were observed to be tended by bulls, Mary Mountain bison subpopulation, Yellowstone National Park, Wyoming, 1990.
Fig. 2 Proportion of lactating and non-lactating bison cows ovulating during the rutting season, Mary Mountain subpopulation, Yellowstone National Park, 1990. Ovulation was based on fecal progesterone concentrations > 3,000 ng/g dry feces or urinary PdG concentrations of > 100 ng/mg Cr.
We tested a total of 85 cows from the MM herd for pregnancy between mid-October and 15 November 1990; 32 (37.6%) were pregnant. Of the 32 pregnancies, 5 (15.6%) were among lactating cows and 27 (84%) were among non- lactating cows. Over 2 years, the combined pregnancy rate for 255 cows from both subpopulations was 48.2%, with 15.4% of the pregnancies among lactating cows (Table 1). Among 12 tended cows from which samples were collected 3-5 days following observed tending, 10 had elevated P 4 concentrations consistent with a luteal phase in bison.
Table 1. Pregnancy rates for non-lactating and lactating bison cows in 2 subpopulations at Yellowstone National Park. Wyoming. 1989-90.
Year |
Subpopulation |
n |
Percent pregnant | |
| Lactating cows | All cows | |||
| 1989 | NRa | 87 | 16 | 57.4 |
| 1990 | NR | 83 | 14.6 | 49.3 |
| 1990 | MMb | 85 | 15.6 | 34.1 |
| aNorthern Range herd. bMary Mountain herd. |
||||
Discussion
In earlier studies, endocrine relationships in bison were not established for reproductive events such as the estrous cycle and early pregnancy. Our data indicate that monitoring ovarian steroid hormone activity during the rutting season, and ovarian and placental steroid production during pregnancy can provide important physiological indices of reproductive function in free-roaming bison. Visual observation of estrous and breeding behaviors can be used to infer ovulation and/or pregnancy in bison (McHugh 1958) and other free-roaming ungulates (Berger 1983). However, in other ungulates estrous behavior and even breeding activity with or without elevated estrogens are not always accompanied by ovulation (Kirkpatrick and Turner 1983). Single service conception rates often are very low in some ungulate species (Ginther 1979, Short et al. 1990) and unreliable for determining pregnancy rates. The high percentage of tended cows that demonstrated endocrine evidence of ovulation in our study indicates that tending behaviors are reliable indicators of ovulation in bison.
The reliability of urinary PdG and fecal P4 to detect occurrence of ovulation already has been established in bison (Kirkpatrick et al. 1990a, 1991a, 1992), domestic cows, and musk- oxen ( Ovibos moschatus) (Desaulniers et al. 1989); however, some error may be introduced as a result of P4 produced by tissues other than the corpus luteum. Significant production of progesterone by adrenal cortical tissue has been demonstrated in fallow deer (Dama dama) (Asher et al. 1989) and white-tailed deer (Odocoileus virginianus) (Plotka et al. 1983).
Among the Yellowstone bison, our visual observations during a 2-year period indicate calving rates between 35 and 55%, which imply an every-other-year or every-3-year pattern of calving. Of lactating cows, 18.5% were witnessed being tended, inferring ovarian activity. With steroid analysis, 14.5% of the lactating cows demonstrated endocrine evidence of ovulation, and 15.6% and 16% demonstrated endocrine evidence of pregnancy. Thus, the physiological data obtained from steroid analysis regarding ovulation and pregnancy among lactating and non-lactating cows are consistent with visual observations. These data indicate that lactating cows with calves have significantly reduced fertility and that lactational suppression of ovarian activity is the primary mechanism by which fecundity is reduced.
The low percentage of lactating bison being tended during the rutting season, and documented as pregnant in autumn, is consistent with reproductive patterns seen in the closely related domestic cow. In the latter, there is a significantly longer interval between parturition and the first estrus than in non-lactating cows (Anderson 1969). This delay in the onset of estrus in domestic cows is caused primarily by suckling, suggesting a classic lactational anestrus (Short et al. 1990), and it is likely that the same phenomenon occurs in bison. Interestingly, the percentage of lactating bison cows being tended increases from July through the end of August, a pattern that suggests that bison experience a lengthy post-partum interval, from which the physiologically inactive ovary begins to recover by late August, as is common to lactating domestic cows.
Research Implications
The remote monitoring of ovulation and pregnancy among bison of YNP indicates that pregnancy generally occurs on alternate years; approximately 15% of lactating cows will become pregnant on consecutive years; the cause of low pregnancy rates in lactating cows is failure to ovulate rather than pre- or neonatal losses; and endocrine evidence of ovulation and pregnancy is consistent with observed reproductive behaviors. The use of urinary and fecal steroid analysis can provide safe, accurate, and non-stressful methods for monitoring reproduction in free-roaming bison. These techniques also can be adapted to other free-ranging wildlife species to accurately estimate reproductive variables in populations where capture is difficult or impossible (Lasley and Kirkpatrick 1991).
Literature Cited
Anderson, L. L. 1969. Sexual behavior and controlling mechanisms in domestic birds and mammals. Pages 541-568 in H. H Cole and P. T. Cupps, eds. Reproduction in domestic animals. Academic Press, New York, N.Y
Asdell, S. A. 1964. Patterns of mammalian reproduction. Cornell Univ. Press, Ithaca, N.Y. 670pp.
Asher, G. W., A. J. Peterson, And D. Duganzich. 1989. Adrenal and ovarian sources of progesterone in young female fallow deer (Dama dama ). J. Reprod. Fert. 85:667-675.
Berger, J. 1983. Induced abortion and social fac- tors in wild horses. Nature 303:59-61.
Desaulniers, D. M., A. K. Goff, K. J. Betferidge, J. Rowell, And P. F. Flood. 1989. Reproductive hormone concentrations in faeces during the oestrous cycle and pregnancy in cattle (Bos taurus) and musk oxen (Ovibos moschatus). Can. J. Zool. 67:1148-1154.
Fuller, W. A. 1959. The horns and teeth as in- dicators of age in bison. J. Wildl. Manage. 23: 342-344.
Ginther, 0. J. 1979. Reproductive biology of the mare. McNaughton and Gunn, Inc., Ann Arbor, Mich. 413pp.
Green, W. C. H. 1986. Age-related differences in nursing behavior among American bison cows (Bison bison). J Mammal. 67:739-741.
Haugen, A. 0. 1974. Reproduction in the plains bison. Iowa State J. Res. 49:1-8.
Kirkpatrick, J. F., K. Bancroft, And V. Kincy. 1992. Pregnancy and ovulation detection in bison (Bison bison) assessed by means of urinary and fecal steroids. J. Wildl. Dis. 28:590-597.
Kirkpatrick, J. F, L. H. Kasman, B. L. Lasley, And J. W. Turner, Jr. 1988. Pregnancy determination in uncaptured feral horses. J. Wildl. Manage. 52 305-308.
Kirkpatrick, J. F , V. Kincy, K. Bancroft, S. E. Shideler, And B. L Lasley 1991a. Oestrous cycle of the North American bison (Bison bison) char- acterized by urinary pregnanediol-3-glucuro- nide. J Reprod. Fert. 93.541-547.
Kirkpatrick, J. F , B. L. Lasley, And S. E. Shideler. 1990a. Urinary steroid evaluations to monitor ovarian function in exotic ungulates. VII. Urinary pro-gesterone metabolites in the Equidae assessed by immunoassay. Zoo Biol. 9:341-348.
Kirkpatrick, J. F., S. E. Shideler, B. L. Lasley, And J. W. Turner, Jr 1991b. Pregnancy diagnosis in fe- ral horses by means of fecal steroid conjugates. Theriogenology 35:753-759.
Kirkpatrick, J. F., J W. Turner, Jr. 1990b. Pregnancy determination in uncaptured feral horses based on steroid metabolites in urine-soaked snow and free steroids in feces. Can. J. Zool. 68. 2576-2579.
Kirkpatrick, J. F., J. W Turner, Jr. 1983. Seasonal pat- terns of LH, progestins and estrogens in feral mares. J. Equine Vet Sci.3.113-118.
Lasley, B. L., And J. F. Kirkpatrick. 1991. Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. J. Zoo Wildl. Med. 22.23-31.
Lott, D. F. 1981. Sexual behavior and intersexual strategies in American bison. z. Tierpsychol. 56 97-114.
Lott, D. F , J. C. Galland. 1987. Body mass as a factor influencing dominance status in American bison cows. J. Mammal. 68.683-685.
Maher, C R., And J. A. Byers. 1987. Age-related changes in reproductive effort of male bison. Behav. Ecol. Sociobiol. 21.91-96.
McHugh, T. 1958. Social behavior of the American buffalo (Bison bison bison). Zoologica 43:1-40.
Meacher, M. M. 1973. The bison of Yellowstone National Park. Natl. Park Serv. Monogr. Ser. No. 1. 161pp.
Meacher, M. M 1989. Range expansion by bison of Yellow- stone National Park. I. Mammal. 70:670-675.
Mostl, E., H. S. Choi, W. Wurm, N. Ismail, And E. Bamberg. 1984. Pregnancy determination in cows and heifers by determination of oestradiol-17o in faeces. Brit. Vet. I. 140:287-291.
Munro, C. I., And C. H. Stabenfeldt. 1984. Development of a microtiter plate enzyme immunoassay for the determination of progesterone. J. Endocr. 101:41-49.
Norland, I. E. 1984. Habitat use and distribution of bison in Theodore Roosevelt National Park. M.S. Thesis, Montana State Univ., Bozeman. 131pp.
Plotka, E. D., R. E. Erb, C. I. Callahan, And W. R. Gomes. 1967. Levels of progesterone in pe- ripheral blood plasma during the estrous cycle of the bovine. I. Dairy Sci. 50:1158-1160.
Plotka, E. D , U. S. Seal, L. I. Verme, And I. I. Ozoga. 1983. The adrenal gland in white-tailed deer: a significant source of progesterone J. Wildl. Manage. 47:38-44.
Rutberg, A. T. 1984a. Competition and reproduction in American bison cows. Ph.D. Thesis, Univ. of Washington, Seattle. 178pp.
Rutberg, A. T.1984b. Birth synchrony in American bison (Bison bison): response to predator or season? J Mammal. 65:418-423.
Rutberg, A. T.1986. Dominance and its fitness consequences in American bison cows. Behaviour 9:62- 91.
Shideler, S. E., L. Tell, G. Owiti, L. Laughlin, R. Chatterton, And B. L. Lasley. 1990. The relationship of serum estradiol and progesterone concentrations to the enzyme immunoassay measurement of urinary estrone conjugates and immunoreactive pregnanediol-3a-glucuronide in Macaca mulatta. Am. I. Primatol. 22:113-122.
Short, R. A., R. A. Bellows, R. B. Stagmiller, I. D. Berardinelli, And E. E. Custer. 1990. Physiological mechanisms controlling anestrus and infertility in postpartum beef cattle J. Anim. Sci. 68:799-816.
TAUSSKY, H. H. 1954. A microcolorimetric determination of creatinine in urine by the Jaffe reaction J. BioI. Chem. 208:853-861. ,
